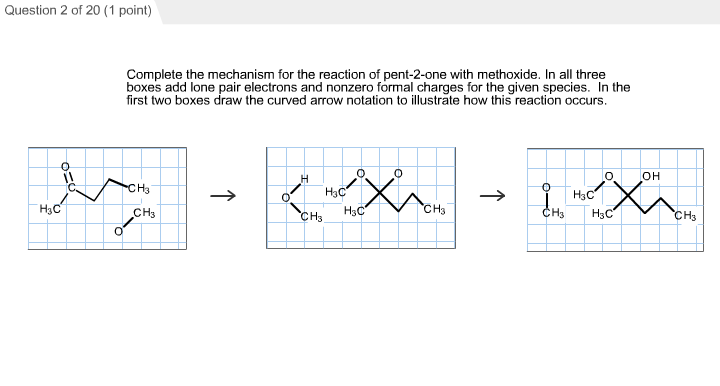
Complete the Mechanism for the Reaction of Pent-2-one
This preview shows page 196 - 198 out of 257 pages. Deduce the name of.

Pentan 2 One An Overview Sciencedirect Topics
277 b Both faces are equally susceptible to attack so the alcohol product is racemic and most hydride reagents discussed.

. Addition of HOCH2CH2OH and H to Penta-2-none Activity 16 Activity 16 Activity 16 Formation of Acetals Tutorial 16 Activity 16 Activity 16 Activity 16 Activity 16 Activity 16 Activity 16 Activity 16 Activity 16 Ketones. Groups that were trans remain trans. The cracking of one molecule of compound X produces pent-1-ene ethene and butane in a 121 mol ratioDeduce the molecular formula of X and state a use for the ethene formed.
When pentan-2-one is reduced to pentan-2-ol for example the carbonyl is a prochiral center Section 145 since addition of hydride from either of the two faces a or b of the carbonyl unit in pentan-2-one will generate the two enantiomers of pentan-2-ol. The cracking of one molecule of compound X produces pent-1-ene ethene and butane in a 121 mol ratio. It is unimolecular two step process.
In the presence of the NADPH-generating system liver. I Name and outline a mechanism for the conversion of 2-bromopentane into pent-2-ene as shown below. Addition of NaCN to 3-methylcyclohex-2-en-1-one Activity 16.
The mechanism if the reaction is given here. Who are the experts. In order to ensure that the oxidation to ethanoic acid is complete the reaction is carried out under reflux.
A 3 1 x -1 Complete the mechanism for the reaction of pent-2-one with methoxide. Groups that were cis in the original alkene remain cis in the product. Up to 24 cash back The following conversions illustrate a number of different types of reaction mechanism.
LOTUS - the natural products occurrence database. The addition is a concerted syn addition. Complete the mechanism for the reaction of Pentan-2-one with methoxide.
The major product will be 3-Bromopentane because the carbocation formed in this case will be more stable than that formed during the formation for 2-Bromopentane. 1 Answer to Complete the mechanism and the products for the reaction of 2-pentene with N-bromosuccinimide NBS in light and carbon tetrachloride. The number of possible stereoisomers for the product is.
And hence the products formed will be. Name and outline the mechanism of reaction 1 halogenoalkane for the formation of pent-1-ene. Use your knowledge of organic reaction mechanisms to complete the mechanism for this step by drawing two curly arrows on the following equation.
The first two chlorinations lead to dichloride 4Cleavage of dichloride 4 with base gives enolate 6. Carbocation intermediate is formed so rearrangement is possible in SN1 reaction. The epoxidation of trans -pent-2-ene produces a racemic mixture of trans -pent-2-ene epoxide isomers.
Methyl propenyl ketone is an enone that is pent-2-ene in which the two methylene hydrogens have been replaced by an oxo group. Pent-3-en-2-one is a natural product found in Cichorium endivia and Tamarindus indica with data available. Find x such that the matrix A is nonsingular.
The metabolites thus obtained were 2-pentanol 3-pentanol and 2-pentanone. IUse your knowledge of organic reaction mechanisms to complete the mechanism for this step by drawing two curly arrows on the following equation. 3-menthyl-pent-2-ene on reaction with HBr in presence of peroxide forms an addition product.
Then their blood and liver tissue were collected and analyzed by means of GC and GC-MS. Male mice of ICR strain were exposed to about 5 n-pentane for one hour while the oxygen in the environmental air was maintained at about 20. Use curved arrows bonds atoms and electrons to complete the mechanism and.
Draw the simplified curved arrow mechanism for the reaction of butan-2-one and CH3Li to give the major product. We review their content and use your feedback to keep the quality high. Up to 24 cash back a One of the steps in the mechanism for Reaction 1 involves the replacement of the functional group by bromine.
Add the appropriate arrows to show the mechanism to form intermediate A. Complete the following reactions and explain optical activity of the products formed. SN1 Reaction of Alkyl halide Mechanism.
Br- H H- H H H A Answer Bank This intermediate A has a resonance structure B as shown. The reaction of toluene with the bromine radical forms a radical A. Ionisation of alkyl halide Slow step rds Characteristics of SN1 reactions.
The first propagation step. A When 2-bromopentane reacts with ethanolic KOH two structurally isomeric alkenes are formed. In the first two boxes draw the curved arrow notation to illustrate how this.
Asked Jun 22 2019. Experts are tested by Chegg as specialists in their subject area. Also need to draw curved arrow notation and add lone pair electrons and nonzero formal charges.
2 6. In all three boxes add lone pair electrons and nonzero formal charges for the given species. I assume that there is sufficient base and chlorine and that the conditions are vigorous enough to get beyond dichloride 4 which being a non-enolic β-diketone at C 3 is labile toward Haller-Bauer-type cleavageAcetylacetone 1 is highly enolic and readily deprotonated.
I Pent-1-ene with HBr. When Pent-2-ene reacts with HBr their is option for H to either attack the 2nd carbon or the 3rd carbon.

Pentan 2 One An Overview Sciencedirect Topics
Solved Complete The Mechanism For The Reaction Of Pent 2 One Chegg Com

Comments
Post a Comment